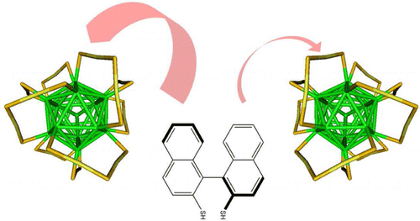-
Structural Information on the Au-S Interface of Thiolate-Protected Gold Clusters: A Raman Spectroscopy Study
B. Varnholt, P. Oulevey, S. Luber, C. Kumara, A. Dass and T. Bürgi
The Journal of Physical Chemistry C, 118 (18) (2014), p9604-9611


DOI:10.1021/jp502453q | unige:37937 | Abstract | Article HTML | Article PDF

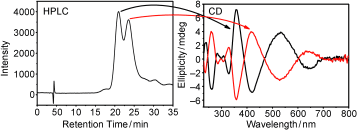
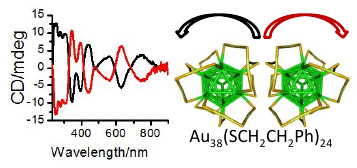
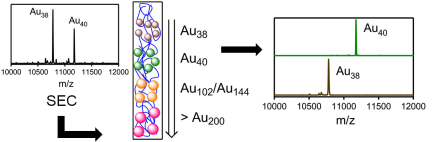
The Raman spectra of a series of monolayer-protected gold clusters were investigated with special emphasis on the Au–S modes below 400 cm–1. These clusters contain monomeric (SR-Au-SR) and dimeric (SR-Au-SR-Au-SR) gold–thiolate staples in their surface. In particular, the Raman spectra of [Au25(2-PET)18]0/–, Au38(2-PET)24, Au40(2-PET)24, and Au144(2-PET)60 (2-PET = 2-phenylethylthiol) were measured in order to study the influence of the cluster size and therefore the composition with respect to the monomeric and dimeric staples. Additionally, spectra of Au25(2-PET)18–2x(S-/rac-BINAS)x (BINAS = 1,1′-binaphthyl-2,2′-dithiol), Au25(CamS)18 (CamS = 1R,4S-camphorthiol), and AunBINASm were measured to identify the influence of the thiolate ligand on the Au–S vibrations. The vibrational spectrum of Au38(SCH3)24 was calculated which allows the assignment of bands to vibrational modes of the different staple motifs. The spectra are sensitive to the size of the cluster and the nature of the ligand. Au–S–C bending around 200 cm–1 shifts to slightly higher wavenumbers for the dimeric as compared to the monomeric staples. Radial Au–S modes (250–325 cm–1) seem to be sensitive toward the staple composition and the bulkiness of the ligand, having higher intensities for long staples and shifting to higher wavenumbers for sterically more demanding ligands. The introduction of only one BINAS dithiol has a dramatic influence on the Au–S vibrations because the molecule bridges two staples which changes their vibrational properties completely.